Pioneering Regenerative Solutions
Merakris Therapeutics is at the forefront of cell-free regenerative medicine. Here, we leverage the extraordinary healing capabilities of human amniotic tissue to develop products that transform the standard of patient care.
The company’s dedicated scientific efforts have led to groundbreaking advancements in regenerative wound care medicine.
Our rigorous research and development efforts have successfully propelled Dermacyte Liquid, an investigational new drug, into Phase 2 clinical trials, marking a pivotal step in bringing our advanced regenerative therapy to patients in need.
Advanced Healing with Cutting-Edge Technology
Our research seeks to answer a fundamental question – what mechanisms underpin the regenerative healing potential of amniotic fluid.
Powered by a brilliant scientific team, Merakris Therapeutics is transforming scientific insights into a new class of cell-free therapeutics for wound care and other unmet clinical needs.
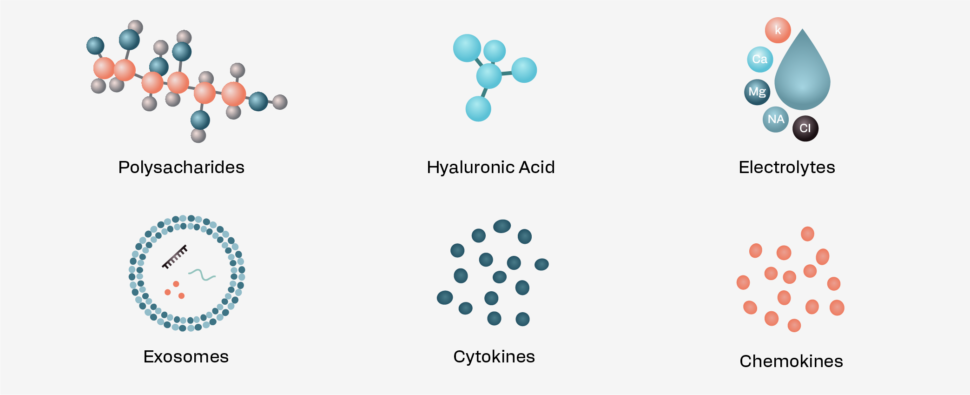
Cell-Free Amniotic Fluid (Dermacyte Amniotic Wound Care Liquid)
Amniotic Fluid (AF), vital for fetal development, is a rich source of regenerative cells.1
The cell-free form of amniotic fluid (cfAF) is also a powerful stimulant of healing and repair. Its immunomodulatory, anti-inflammatory and pro-growth characteristics make cfAF a valuable agent in wound healing.2
Our proprietary tissue engineering technologies involve the isolation of cfAF under the highest safety standards, while preserving the natural properties of amniotic fluid.
By harnessing the secreted proteins and signaling molecules of amniotic fluid cells, we have created a first in class cell-free solution currently in Phase II clinical trials.3
This technology represents a first-in-class approach to precision wound healing by activating epithelial to mesenchymal transition pathways necessary to support early-stage wound healing then mesenchymal to epithelial transition to support late-stage healing and keratinization.4

Select Proteins from qELISA Analysis of Dermacyte Liquid (data on file)
Co-cultured Amniotic Stem Cell Conditioned Media (coACCM)
Merakris scientists are currently working to develop a revolutionary therapeutic solution – a meticulously engineered in vitro amniotic stem cell co-culture system.
This innovative system cultivates two distinct cell types isolated from amniotic fluid cells and amniotic epithelial cells. The two cell types undergo independent expansion before being combined in a co-culture system. Within this controlled system, which mimics the in utero environment, the cells interact and communicate to trigger the secretion of a rich array of biomolecules including growth factors and cytokines that are crucial for regeneration.
Advantages of coACCM
Consistent and enriched source of therapeutic factors: coACCM overcomes the variability associated with donor-derived amniotic fluid and ensures a consistent supply of potent therapeutic factors.
This technology will allow us to tailor treatments to specific wound types and stages, marking a significant step towards personalized medicine in wound care and beyond.
Scientific Publications
Cell-Free Amniotic Fluid and Regenerative Medicine: Current Applications and Future Opportunities
Comparative Analysis of Co-Cultured Amniotic Cell-Conditioned Media with Cell-Free Amniotic Fluid Reveals Differential Effects on Epithelial-Mesenchymal Transition and Myofibroblast Activation
Safety and efficacy of acellular human amniotic fluid and membrane in the treatment of non-healing wounds in a patient with chronic venous insufficiency
Adaptive Design Clinical Trial: Dermacyte® Amniotic Wound Care Liquid for the Treatment of Non-Healing Venous Stasis Ulcers (VSU)
Our current focus is on the clinical trial of Dermacyte Amniotic Wound Care Liquid (an investigational drug) in the treatment of non-healing chronic venous leg ulcers/diabetic foot ulcers (VLU/DLU) as a novel subcutaneous therapy.
Our future endeavors include expanding the scope of our technologies beyond wound care and other undisclosed targets.
Charting the Future of Regenerative Medicine


References:
- Shamsnajafabadi H, Soheili ZS. Amniotic fluid characteristics and its application in stem cell therapy: A review. Int J Reprod Biomed. 2022 Sep 6;20(8):627-643.
- Bowen CM, Ditmars FS, Gupta A, Reems JA, Fagg WS. Cell-Free Amniotic Fluid and Regenerative Medicine: Current Applications and Future Opportunities. Biomedicines. 2022 Nov 17;10(11):2960.
- A Two Part, Randomized Study of Dermacyte® Amniotic Wound Care Liquid for the Treatment of Non-Healing Venous Stasis Ulcers. ClinicalTrials.gov Identifier: NCT04647240. U.S. National Library of Medicine, 2020. https://classic.clinicaltrials.gov/ct2/show/NCT04647240 (accessed 2024-03-16).
- Liu N, Bowen CM, Shoja MM, Castro de Pereira KL, Dongur LP, Saad A, Russell WK, Broderick TC, Fair JH, Fagg WS. Comparative Analysis of Co-Cultured Amniotic Cell-Conditioned Media with Cell-Free Amniotic Fluid Reveals Differential Effects on Epithelial–Mesenchymal Transition and Myofibroblast Activation. Biomedicines. 2022; 10(9):2189.